A colloidal substance is any which has the quality of having another substance diffused evenly throughout it.
In the example of milk, there is a dispersal of liquid butterfat within a water-based solution. In this case, the liquid butterfat, the smaller molecule, is within the range of 1 nanometer and 1 micrometer, making it invisible to an optical microscope. When the mixture becomes homogenous, that is to say that no part of the solution has a greater or lesser concentration of the dispersed phase than any other, it can be said that the solution is colloidal in nature. The larger solution that contains the dispersed phase is called the continuous phase, or the dispersion medium, since its size and distribution does not change, while the dispersed phase spreads throughout it.
Types of colloids include colloidal aerosols, colloidal emulsions, colloidal foams, colloidal dispersions, and mixtures called hydrosols. Every colloidal system has two phases: the dispersed phase, and the continuous phase.
Colloids Through History
Intense interest in colloidal substances was present throughout the sciences in the early 20th century. Einstein and others studied their characteristics and applications closely. At the time, this emerging field of science was a leading area of inquiry for theorists, researchers, and manufacturers.
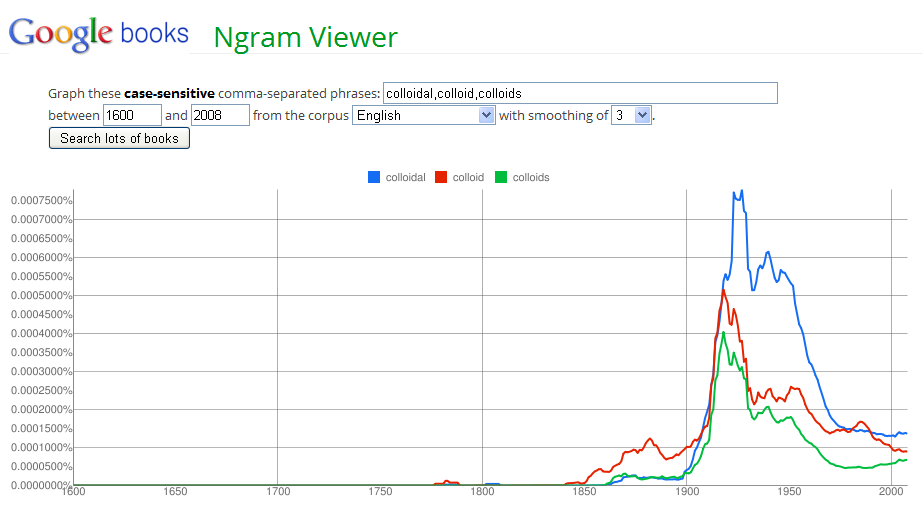
The image above shows the history of publications surrounding colloids. This entire history is relatively recent. After a peak in interest prior to 1950, the study of colloids decreased greatly. To enlarge the image, just click on it.
Interestingly, with the recent dawn of higher-powered microscopes and “nano-technology” (the study of objects of a certain tiny scale), there is once again a growing scientific interest in exploring new materials that incorporate tiny bits of matter along with their potential new applications. While colloids and nano-technology are not the same thing, there is much overlap between these fields of study.
Colloidal Substances
There are substances both observed in nature and in manmade solutions which have colloidal properties. For example, mayonnaise, cosmetic lotion, and lubricants are types of manmade emulsions, while milk is a colloidal emulsion that occurs in nature. To go further, colloidal foams include whipped cream and shaving lather, while edible colloids include butter, marshmallow substance, and jelly. In addition to colloids in food, like the above, colloids exist in the form of some alloys, paint, ink, detergents, insecticide, aerosol sprays, styrofoam, and rubber. Even beautiful natural substances such as clouds, pearls, and opals have colloidal properties, because they have another substance evenly distributed through them. The most common colloidal substance bearing the name “colloidal” is colloidal silver, a combination of tiny pieces of silver within water.
By increasing small molecules to the range of range of between 1 nanometer and 1 micrometer, or by reducing large molecules to the same size, colloidal substances can be prepared. Further production of colloidal substances depends on the type of substances used in the dispersed and continuous phases. Colloids behave differently than normal fluids, and this is generally observed in transport and physico-chemical attributes. For example, a membrane might allow a true solution, that is to say a solution with a solid molecules attached to liquid molecules, to pass through it, while a colloidal substance that has a solid dispersed through a liquid will be strained out by the membrane, since the solids are merely dispersed and not attached.
The evenness of the distribution of the dispersed substance is homogenous to the point of microscopic equality in spacing throughout the second substance.
Topics Covered on This Website
Several of the public’s most commonly asked questions about colloidal substances are addressed on this website. These questions frequently center on the use of colloidal metals as home remedies, but other questions and applications are introduced as well. To browse these topics, use the menu on the side of your screen or choose from these popular topics:
There are way too many topics relating to colloidal substances to cover any these topics exhaustively, so this website is intended as an introductory resource only. It is certainly not to be used as a medical text, lab journal, or how-to-guide. It is simply an introduction to a varied and often misunderstood group of materials.
For more detailed information about colloidal substances, there are countless places to look. Scientific journals are a good source of up-to-date information, though the word “colloidal” may or may not be a part of the article titles.